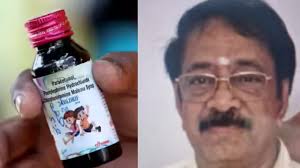
Lagatar24 Desk
Madhya Pradesh: A district court has denied bail to paediatrician Dr Praveen Soni, arrested in the Coldrif cough syrup deaths case, after police informed the court that he confessed to receiving a 10% commission from the pharmaceutical company for prescribing the medicine. The syrup has been linked to multiple child deaths and cases of kidney failure.
Commission Kickbacks And Fatal Outcomes
During police interrogation, Dr Soni admitted to taking a 10% cut from the company for promoting Coldrif, even as reports of children suffering from severe urine retention and kidney issues emerged. At least seven children have died and six others remain under treatment in Nagpur due to complications linked to the syrup. Despite knowing it was unsuitable for children under five, Dr Soni prescribed the medicine between 24 August and 4 October. The first child death occurred on 29 August, followed by another on 5 September with similar symptoms of acute kidney failure.
Violation Of Health Ministry Guidelines
The court cited Union Health Ministry’s 2023 guidelines prohibiting the prescription of Fixed Dose Combination (FDC) medicines like Coldrif for children below four years. It observed that Dr Soni’s continued prescriptions in defiance of these directions were serious and constituted prima facie evidence of negligence and ethical breaches.
Court Rejects Doctor’s Defence
Dr Soni’s counsel argued that Coldrif had been in use for over 15 years and that the doctor prescribed it in good faith without knowing it contained toxic substances. However, the court rejected this defence, emphasizing the gravity of the charges and the number of fatalities as justification for continued custody during investigation.
Toxic Chemical Detected, Probe Widens
Lab tests confirmed the presence of ethylene glycol — a toxic compound — in Coldrif syrup samples, prompting an FIR on 4 October. Police are now tracking the supply chain, company officials, and other doctors involved in the alleged kickback scheme. The Madhya Pradesh government has imposed a statewide ban on the sale and use of Coldrif as part of a broader crackdown on unsafe paediatric medicines.